I’ve attended several Life Sciences events recently (including Biomanufacturing Summit) and it’s quite clear that these supply chain teams are working in a new, complex world. Not only do they need to meet diverse customer expectations, but they need to do so while coordinating an extended supply chain, in an environment that is constantly changing. Additionally, they’re faced with a set of five industry trends that are driving complexity even further.
1. Exceedingly Distinct Markets
Through accidents of history and industrial capabilities, the Life Sciences industry has developed to satisfy principally the diseases of the affluent West, such as cardiovascular disease, diabetes, respiratory disease, and obesity, while paying less attention to the diseases prevalent in the developing world, such as malnutrition, malaria, HIV/AIDS, and TB. This has led to a drug market segmented by geography and demographics, with companies in the emerging markets focused on satisfying the ‘local’ diseases. But in recent years, with the rapid expansion of the middle class in many emerging economies, many of the ‘Western’ diseases are increasing rapidly in the middle classes of the emerging markets – for example diabetes in India – stretching local healthcare provision while opening opportunities for expansion into these countries. While at the same time innovations by companies in emerging markets are challenging the market leadership of well-established Life Sciences companies in the West.
2. Increased Outsourcing
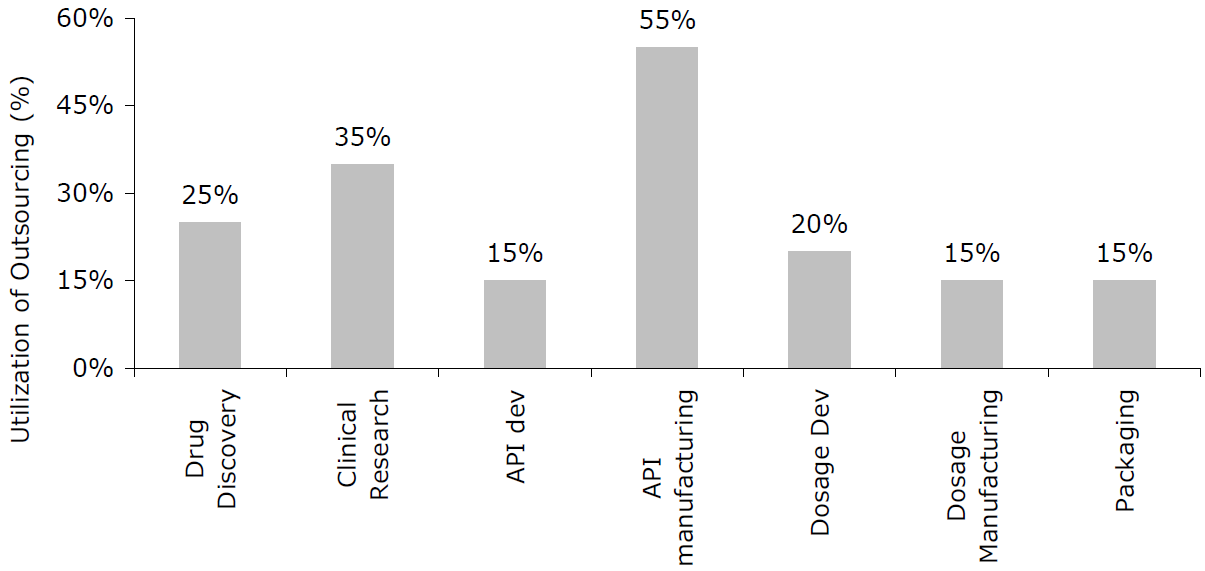
With tremendous opportunities for growth in emerging markets, many manufacturers have executed aggressive globalization and outsourcing strategies, while relying increasingly on Third Party Operators (TPOs) in India and China for Active Pharmaceutical Ingredient (API) supply and subcomponents, or even the manufacturing of complete devices. Coming along with these shifts is an increase in business complexity and supply chain risks given the varying regulations across global supply chains and longer and riskier supply chains.
3. New Regulations
With this rapid increase in the use of TPOs has come added risks to quality and of counterfeiting, leading the US Food and Drug Administration (FDA) to push for the passage of the Safety and Innovation Act (FDASIA), which focuses on the risks inherent in an increasingly global Life Sciences supply chain. Much of the public comment has been on the two user fee reauthorizations, as well as two new user fee programs, and the reauthorization for pediatric research. But buried deep in the text are provisions for supply chain validation – in both domestic and off-shore plants – and drug shortages that will have a profound impact on outsourced and global supply chains. Stefanie Johns, Ph.D., Program Manager, Xavier Health Initiatives, commenting on conference sessions at Xavier University, states that: “The new powers from FDASIA will level the playing field between foreign and domestic sites, enhance transparency and collaboration with foreign regulators, and shift focus “away from the border to a global safety net.” FDASIA also provides the FDA with new tools to destroy counterfeit products, misbrand products on the basis of inspection refusal, and deliver criminal penalties for intentional adulteration. In order to streamline resources, the FDA will be moving towards a risk-based inspection system and will work with foreign regulatory counterparts.” In summary, the impact of FDASIA on the Life Sciences supply chain will come from provisions for:
- reporting of drug shortage issues, and the penalties associated with not informing the FDA;
- and more active inspections of production facilities, including sites in other countries, including those belonging to Third Party Operators.
Outsourcing in the Pharmaceutical Industry
Source: Frost and Sullivan Global Bio-Pharma CMO Market Report,“ May 2010
4. Shift in Treatment Focus One side effect of FDASIA is the fast-tracking of approval for treatments that address an ever narrower spectrum of diseases. Of particular importance to rare disease patients, and likely to help encourage further investment, is the Breakthrough Therapies Act addressing the need to provide expedited development and evaluation of potential therapies that show promise early in the research process; and the Therapeutics for Rare and Neglected Diseases which aims to encourage and speed up the development of new drugs for rare and neglected diseases. Included in the Breakthrough Therapies Act is a voucher system that allows companies developing rare pediatric diseases to obtain a transferable voucher which they can use for the expedited approval of another treatment, whether that treatment satisfies the requirements for priority review or not. The trend to ever more targeted products is widespread across most industries whether Life Sciences, High-Tech/Electronics, or Consumer Goods. In the past, the limited markets coupled with the fact that many of the patients were in less affluent areas of the world, were a disincentive to major Life Sciences companies that were addressing a large set of diseases with broad spectrum therapeutics. However, with many of the major disease categories covered effectively by existing treatments, combined with the fact that a) many treatments are reaching the end of their patent protection period, b) growing competition from generics, and c) increasing scrutiny from regulatory bodies have all led to a rapid shift in focus of research, as well as mergers and acquisition activity toward rare diseases. (While there isn’t a universally accepted definition of a rare disease, the US government defines a rare disease as one afflicting fewer than 200,000 Americans, while the European Union defines a rare disease as one afflicting fewer than 1 in 2,000 people.)
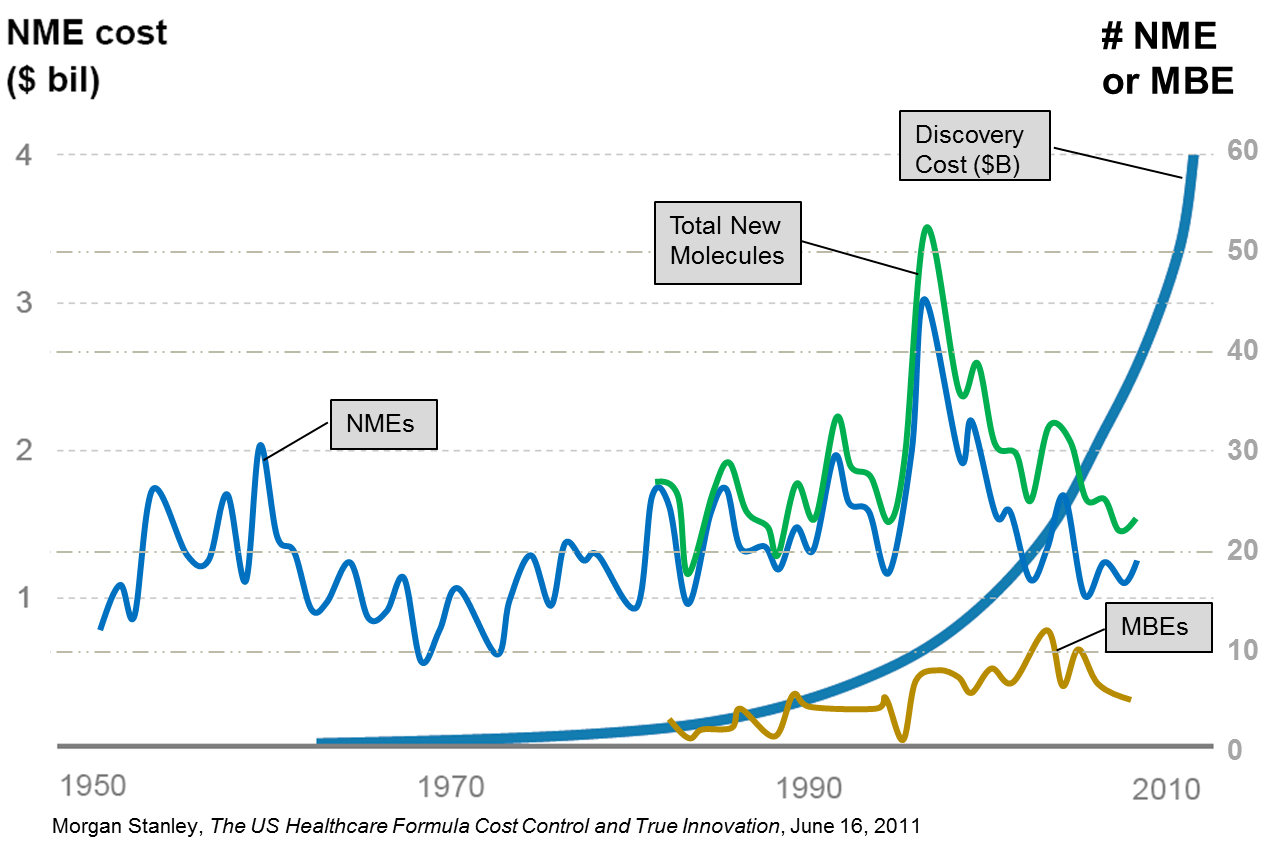
Innovation versus Cost
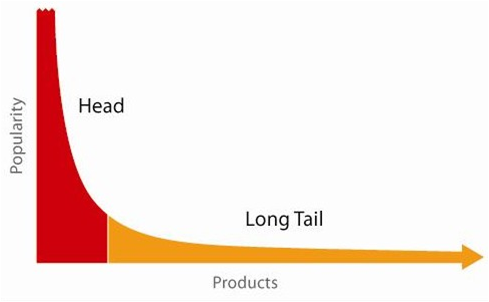
A report released by the Pharmaceutical Research and Manufacturers of America (PhRMA) in 2011 emphasized the extent of this shift away from broad spectrum drug research focused on diseases with large patient bodies to narrow spectrum drugs focused on rare diseases. According to the PhRMA report there were a record 460 medicines for rare diseases either in clinical trials or awaiting FDA review at the time the report was published. To overcome the economic barriers associated with the discovery and development of diagnostic equipment, drugs and devices to treat rare disease, big Life Sciences companies have been pursuing collaborations, acquisitions, and joint ventures, often with companies in India and China. This search for ‘long tail’ drugs will mean that Life Sciences must also deal with increasingly complex demand patterns. They have to simultaneously deal with predictable patterns for mid-life cycle products and highly unpredictable patterns for new introductions. They typically have to manage both low volume, high mix products that require quick response for clinical trials and high volume products that require ramped production and global delivery capabilities.
5. Shorter Patent Protection An aging product portfolio, along with a future of shorter patent periods in general, with limited opportunities for patent extensions (as demonstrated by the recent challenge by the Indian government of patent extensions based upon reformulation), only serves to reinforce the critical requirement for supply chain efficiency and effectiveness, in order to capitalize fully on the opportunities while they exist. These industry trends are having a significant impact on the way supply chains must operate. And unfortunately, there is growing evidence that existing technology architectures are not satisfying the capability needs for this new, complex world. In an upcoming blog post, I’ll be looking at seven supply chain processes (including jurisdictional control, expiry management, supply and capacity planning) that require an integrated approach to overcome these complexity drivers.




Leave a Reply